Chapter 2: Solutions
What is a Solution?
Solutions are a part of our daily lives because they can be found in almost everything we use in our daily lives, such as soda, deodorant, sugar, salt, and so on. A solution is a type of mixture in which two or more substances combine to form a single solution; it can also be described as simple, and its properties may or may not have changed.
Here in this article, the concept, components, properties, types of solutions based on their nature, and so on. As this is one of the basic concepts from Chapter Solution, this article will help to have a strong foundation in chemistry, to study it in higher classes. So, Let’s get started!
Solution Definition
Solution is defined as,
A solution is a mixture of two or more components that is homogeneous. The makeup of a homogeneous mixture is consistent throughout. The true solution is also known as the homogeneous solution.
True solutions have dissolved particles that are the same size as molecules. As a result, true solutions are referred to as molecular solutions. True solutions are formed only by soluble substances.
Filtration will not be able to separate the solute from the solution once it has been generated. A light beam is not permitted to pass through the solution. The solute particles inside the solution are invisible to the naked eye. A solution is a single-phase system that is stable.
Components of a Solution
There are two parts in a homogeneous combination, the solute and solvent combine to form any solution. A solute is a substance in a solution that has a lower concentration and dissolves in the solvent, which has a higher concentration than the solute. The final state of homogeneous solutions is usually determined by the state of the solvent, though the state of the solute makes no difference in the solution as long as they are soluble in the solvent.
- Solvent: A solvent is a large amount of substance present in a solution that dissolves solute in it. The solvent is also the medium of the solution. Some solvents are Water, Ethanol, Ethyl acetate, Acetone, Benzene, etc.
- Solute: Solute is the substance that is present at a minimum amount according to the need and that gets dissolved in the solvent. Some of the names of solutes are Salt in water, Sugar in tea, Oxygen in seawater, Zinc in dental amalgam, etc.
Types of Solutions
There are various types of solutions based on various parameters, some of these types are:
Based on Solvent
Based on the type of solvent, a solution is classified as aqueous and non-aqueous.
- Aqueous solution: The solution which contains water as its solvent is known as an aqueous solution. For eg; A mixture of sugar and water, hydrogen and water, etc.
- Non-Aqueous solution: The solution which doesn’t contain water as its solvent is known as a non-aqueous solution. For eg: a mixture of gold and copper, sand and salt, etc.
Based on Dissolved Solute
Based on the amount of solute dissolved a solution is classified as saturated, unsaturated, and supersaturated.
- Unsaturated solution: An unsaturated solution is defined as a solution in which a solvent can dissolve less amount of solute in it at a given temperature. For eg: a vinegar solution.
- Saturated solution: A saturated solution can be defined as a solution in which the solvent can dissolve more amount of solute in comparison to the unsaturated solution in it at a given temperature. For eg: a sugar solution.
- Supersaturated solution: A supersaturated solution is defined as a solution that consists of a large amount of solute in it at a given temperature where the extra will be reduced and crystallized quickly. For eg: a mixture of sodium acetate and water.
Based on the Amount of Solvent
Based on the amount of solvent used a solution is classified as dilute solution and concentrated solution.
- Dilute solution: The dilute solution can be defined as the solution in which less amount of solute is dissolved in a large amount of solvent. For eg: salt solution, sugar solution, etc.
- Concentrated solution: The concentrated solution can be defined as the solution in which a large amount of solute is dissolved in less amount of solvent. For eg: orange juice, dark-colored tea, etc.
Based on the Concentration of Two Solutions
While comparing two solutions, we can classify the solution as hypertonic, hypotonic, and isotonic solutions.
- Hypertonic Solutions: In two solutions, whichever of the solution have a higher concentration of solutes (particles) compared to another solution, is called Hypertonic Solutions. Examples of hypertonic solutions include concentrated saline solutions or solutions with high sugar concentrations.
- Hypotonic Solutions: Out of two solutions that are under observation, a solution with a lower concentration of solutes compared to another solution is called a Hypotonic Solution. Distilled water is a common example of a hypotonic solution.
- Isotonic Solutions: If both solutions have similar concentrations of the solutes, then the solutions are called Isotonic Solutions. Normal saline solution (0.9% NaCl) is often used as an isotonic solution in medical settings.
Based on the Uniformity of Solution
Based on the phase uniformity of the solution, we classify a solution as homogeneous and heterogeneous.
- Homogeneous Solution: The solutions in which the composition and phase of solute and solvent are the same throughout, then the solution is called Homogeneous Solutions. For Example, salt dissolved in water.
- Heterogeneous Solution: The solution in which the composition and phase of solute and solvent are distinct is called a heterogeneous solution. Examples include oil dissolved in water etc.
Based on the Number of Components
Based on the number of components used in the solution, we classify a solution as binary, ternary, or quaternary solution.
- Binary solution: A binary solution has only two components one solute and one solvent. Example sugar solution in water.
- Ternary Solution: A ternary solution has three components. Generally, it has two solutes and one solvent. Examples include ORS solution which we make at home contains salt and sugar as solutes and water as a solvent, thus making it a ternary solution.
- Quarternary Solution: A Quarternary Solution has four components in it. Examples include a solution of salt, sugar, lemon, and water. Here we have four components thus making it a Quarternary Solution.
Homogeneous and Heterogeneous Solutions
Homogeneous solutions have uniform composition throughout, while heterogeneous solutions have a non-uniform composition with distinct phases. The following table shows the difference between both types of solutions:
Homogeneous Solutions | Heterogeneous Solutions |
|---|---|
| Uniform composition throughout | Non-uniform composition with distinct phases |
| Components are evenly mixed and cannot be visually distinguished | Components can be visually distinguished |
| Only one phase is present | Multiple phases are present |
| Examples: saltwater, air | Examples: oil and water mixture, salad dressing |
Other Types of Solutions
Any state of matter (solid, liquid, or gas) can act as both a solvent and a solute during the formation of a solution. As a result, there are nine different types of solutions depending on the physical states of the solute and solvent as,
Gaseous Solution
This is the type of solution in which the solvent is present in the gaseous state. It can be categorized into three types on the basis of the solute present in it:
- Gas-Gas Solution: The solution in which both the solute and solvent are present in the gas state is called a gas solution. eg: a mixture of nitrogen and oxygen, carbon dioxide and oxygen, etc.
- Liquid-Gas Solution: The solution in which the solute is present in the liquid state is called a liquid gas solution. eg: a mixture of nitrogen gas and chloroform.
- Solid-Gas Solution: The solution in which the solute is present in the solid state is s called a solid gas solution. eg: a mixture of nitrogen gas and camphor.
Liquid Solution
This is the type of solution in which the solvent is present in the liquid state. It has three types according to the solute present in it:
- Liquid-Gas Solution: The solution in which the solute is present in a gas state is called a liquid gas solution. eg: a mixture of oxygen and water.
- Liquid-Liquid Solution: The solution in which both the solvent and solute are present in the liquid state is called a liquid-liquid state. eg: a mixture of ethanoic acid and water also known as vinegar solution.
- Liquid-Solid Solution: The solution in which the solute is present in a solid state is called a liquid-solid solution. eg: a mixture of sugar and salt.
Solid Solution
This is the type of solution in which the solvent is present in a solid state is called a solid solution. It can also be categorized into three types:
- Solid-Gas Solution: The solution in which solute is present in a gas state is called a solid-gas solution. eg: a mixture of palladium and hydrogen.
- Solid-Liquid Solution: The solution in which solute is present in a liquid state is called a solid-liquid solution. eg: a mixture of salt and water.
- Solid-Solid Solution: The solution in which both the solute and solvent are present in a solid state is called a solid-solid solution. eg; a mixture of silver and gold.
The table summarising the above nine types of solution based on the state of solute and solvent is tabulated below:
S.No | Types of Solution | Solute | Solvent | Examples |
|---|---|---|---|---|
| 1 | Solid-solid | solid | solid | Alloys like brass, bronze, etc. |
| 2 | Solid-liquid | solid | liquid | The solution of sugar, salt, etc in water. |
| 3 | Solid-gas | solid | gas | Sublimation of substances like iodine, camphor, etc into the air. |
| 4 | Liquid-solid | liquid | solid | Hydrated salts, mercury in amalgamated zinc, etc. |
| 5 | Liquid-liquid | liquid | liquid | Alcohol in water, benzene in toluene |
| 6 | Liquid-gas | liquid | gas | Aerosol, water vapor in the air. |
| 7 | Gas-solid | gas | solid | Hydrogen absorbed in palladium |
| 8 | Gas-liquid | gas | liquid | Aerated drinks |
| 9 | Gas-gas | gas | gas | A mixture of gases, etc |
Concentration of a Solution
The composition of solutions can be described by going through their concentration which can be expressed qualitatively or quantitatively. Most of the solutions are determined quantitatively in real life. There are some formulas that can be used to find out whether the solution is dilute or concentrated, that are:
- Mass Percentage
- Volume Percentage
- Mole Fraction
- Molality
- Molarity
- Parts Per Million
Mass Percentage
Mass percentage is also called weight by weight concentration of solute, Mass Percent is defined as the amount of solute (in grams) present in 100 gm of the solution.
Mass Percentage = (Mass of the component in the solution / Total mass of the solution) × 100
Volume Percentage
Volume Percentage is also called volume by volume concentration of solute. It is defined as the amount of solute (in ml) present in 100 ml of the solution.
Volume Percentage = (Volume of the component / Total volume of the solution) × 100
Mole Fraction
Mole Fraction is the ratio of the number of moles of one component to the total number of moles present in the solution.
Mole Fraction of Component = (Number of moles of the components / Total number of moles of all components) × 100
Molarity
Molarity of a solution refers to the number of moles of solute dissolved per liter of solvent. The formula for molarity is given as
Molarity = Numbers of moles of solute/ Volume of solution (in liters)
Molality
Molality of a solution refers to the number of moles of solute dissolved in per kilogram of solvent. The formula for morality is given as
Molality = Number of moles of solute/ Mass of Solvent (in Kg)
Parts Per Million
Parts per Million means the number of parts of solute present per 1 million parts of the solution. Parts per Million is used to measure the concentration of quantities present in trace amounts. For example, if you have to calculate the presence of a mineral in seawater then the concept of PPM is used.
PPM = (Number of Parts of solute/ Total Number of parts of Solutions) × 106
Read More,
FAQs on Types of Solutions
Q1: What are the Different Types of Solutions?
Answer:
Solutions can be classified on the basis of various different parameters, as follows:
- Based on Solvent
- Aqueous solution
- Non-Aqueous solution
- Based on Dissolved Solute
- Unsaturated Solution
- Saturated Solution
- Supersaturated Solution
- Based on the Amount of Solvent
- Dilute Solution
- Concentrated Solution
- Based on the Concentration of Two Solutions
- Hypertonic Solutions
- Hypotonic Solutions
- Isotonic Solutions
- Based on Uniformity of Solution
- Homogeneous Solution
- Heterogeneous Solution
- Based on Number of Componets
- Binary Solution
- Ternary Solution
- Quartenary Solution
Q2: What is a Homogeneous Solution?
Answer:
A homogeneous solution, also known as a homogeneous mixture or a true solution, is a mixture where the solute is uniformly distributed throughout the solvent at the molecular level.
Q3: What is a Heterogeneous Solution?
Answer:
A heterogeneous solution is a mixture where the solute particles are not uniformly distributed throughout the solvent.
Q4: What is the difference between Homogeneous and Heterogeneous Solutions?
Answer:
Homogeneous solutions have uniform composition throughout, while heterogeneous solutions have non-uniform composition with distinct phases.
Q5: What is an Alloy?
Answer:
An alloy is a homogeneous mixture of two or more metals or a metal with a non-metal.
Q6: What is a Solute?
Answer:
A solute is a substance that gets dissolved in a solvent to form a solution. It is the component present in a smaller quantity in the solution.
Q7: What is a Solvent?
Answer:
A solvent is a substance that makes the solute dissolve in it to form a solution. It is the component present in a larger quantity in the solution.
Q8: What is a Dilute Solution?
Answer:
A dilute solution is a solution that contains a relatively small amount of solute dissolved in a solvent. The concentration of the solute is low compared to the amount of solvent.
Q9: What is a Concentrated Solution?
Answer:
A concentrated solution is a solution that contains a relatively large amount of solute dissolved in a solvent. The concentration of the solute is high compared to the amount of solvent.
Q10: How can we determine the Concentration of a Solution?
Answer:
You can determine the concentration of a solution with the concepts of Mass Percentage, Volume Percentage, Mole fraction, Molarity, Molality and Parts Per Million. Refer the sections in this article for detailed formula.
Solubility
Solubility is a fundamental concept in chemistry that describes the ability of a substance to dissolve in a particular solvent under specific conditions to form a solution. A fluid may or may not dissolve completely in a fluid. Understanding the concept of solubility is essential in many fields of science, including pharmaceuticals, environmental science, and materials science.
In this article, we will explore the key concepts of solubility, such as factors that affect solubility, solubility product, and solubility of different phases of matter with each other.
Table of Content
What is Solubility?
Solubility of any solvent is the maximum amount of the solute that can be dissolved in any solvent at any certain temperature. Suppose if we dissolve sugar in the water then the amount of sugar that can be dissolved in water at any given temperature defines the solubility of sugar in water.
Solubility Definition
Solubility refers to the greatest amount of solute that can dissolve in a known quantity of solvent at a given temperature.
In a solvent, a solution is a homogeneous mixture of one or more solutes. A common example of a solution is sugar cubes added to a cup of tea or coffee. Solubility is the property that allows sugar molecules to dissolve. As a result, solubility can be defined as the ability of a material (solute) to dissolve in a specific solvent. Any substance dissolved in a solvent, whether solid, liquid, or gas, is referred to as a solute.
Solubility Effects on Reactions
If we dissolve any solute in a solvent then there are three possible result that are,
Dilute Solution: If a solvent has less solute dissolve in forming the solution then this solution is called the Dilute Solution.
Saturated Solution: If a solvent has maximum solute(that can be dissolved without changing the temperature) dissolve in forming the solution then this solution is called the Saturated Solution.
Precipitate Solution: If a solvent we add excess solute then its saturation limit then it forms the solution in which precipitate is formed also called precipitate solution.
Solubility Product
“Solubility product” refers to salts that are only sparingly soluble. It is the maximal product of the molar concentration of the ions produced by the dissociation of the molecule (raised to their proper powers).
The solubility product remains constant at a given temperature. The lower the value of the solubility product, the lower the solubility, and the higher the value of the solubility product, the greater the solubility. The elements that influence solubility vary depending on the condition of the solute:
- Solubility of Liquids In Liquids
- Solubility of Solids In Liquids
- Solubility of Gases In Liquids
Now let’s learn about the same in detail.
Solubility of Liquids In Liquids
Water is referred to as a universal solvent since it dissolves practically all solutes, with the exception of a few. A substance’s solubility can be influenced by a number of circumstances.
Solubility refers to the development of a new bond between the solute and solvent molecules. Solubility is the highest concentration of solute that dissolves in a known concentration of solvent at a particular temperature in terms of quantity. Solutes are classified as highly soluble, sparingly soluble, or insoluble based on the concentration at which they dissolve in a solvent. It is stated to be soluble if a concentration of 0.1 g or more of a solute can be dissolved in a 100 ml solvent. It is considered to be sparingly soluble when a concentration of less than 0.1 g is dissolved in the solvent. As a result, solubility is defined as a quantitative expression measured in grams per liter (g/L).
Different sorts of solutions can be obtained based on solubility. At a given temperature, a saturated solution is one in which a given amount of solute is entirely soluble in a solvent. A supersaturated solution, on the other hand, is one in which the solute begins to salt out or precipitate once a specific concentration is dissolved at the same temperature.

Factors Affecting Solubility of Liquid in Liquid
The factors affecting the Solubility of Liquid in Liquid are discussed below,
Effect of Pressure
Pressure has a significantly greater impact on gases than it does on solids and liquids. When a gas’s partial pressure rises, so does the likelihood of its solubility. CO2 is bottled under high pressure in a soda bottle, for example.
Effect of Temperature
People can boost a solute’s solubility characteristic by adjusting the temperature. At 20° C or 100° C, water generally dissolves solutes. Increased temperature will totally dissolve sparingly soluble solid or liquid compounds. However, in the case of a gaseous substance, temperature affects solubility in the opposite direction, meaning that as the temperature rises, gases expand and escape from their solvent.
Solubility of Solids In Liquids
Solid solubility has been observed to be dependent on both the composition of the solute and the solvent. People frequently see that some substances, such as sugar and common salt (NaCl), dissolve quickly in water whereas others, such as naphthalene, do not. Only polar solutes prefer to dissolve in polar solvents, while non-polar solvents dissolve only in non-polar solutes, according to different observations and experimental data. As a result, one of the most important elements impacting solubility is the composition of the solvent. The discovery that like dissolves like led to the conclusion that polar solvents dissolve polar solutes and non-polar solvents dissolve non-polar solutes.
Let’s take a closer look at how a solid dissolves in a solvent. Dissolution occurs when a solid solute is given to a solvent and the solute particles dissolve in the solvent. The process of crystallization occurs when solute particles in a solution clash with one another and some of the particles separate from the solution.
Between these two processes, a state of dynamic equilibrium is formed, at which point the number of solute molecules entering the solution equals the number of particles exiting the solution. As a result, at a given temperature and pressure, the concentration of the solute in the solution will remain constant.
A saturated solution is one in which no more solute can dissolve in the solvent at a given temperature and pressure, and it contains the maximum amount of solute. Solubility refers to the concentration of a solute in a solution at a certain temperature and pressure. An unsaturated solution is one in which more solute can be added to the solution.

Factors Affecting Solubility of Solids In Liquids
The factors affecting the Solubility of Solids in Liquid are discussed below,
Effect of Temperature
If (∆solH > 0), the solubility of a nearly saturated solution increases as the temperature rises, and if (∆solH < 0), the solubility falls as the temperature rises.
Nature Solute and Solvent
Like disintegrates into like. Anthracene, for example, does not react with sodium chloride. Naphthalene and anthracene, on the other hand, dissolve quickly in benzene, whereas sodium chloride and sugar do not.
Effect of Pressure
Changes in pressure have little effect on solid solubility. This is owing to the fact that solids and liquids are highly incompressible and are essentially unaffected by pressure fluctuations.
Solubility of Gases In Liquids
The topic of gas solubility in liquids is concerned with the idea of gas dissolving in a solvent. Let’s start with a definition of solubility. Solubility is the greatest amount of solute that may be dissolved in a given solvent at a given temperature for any substance. Our current interest is the solubility of gases in liquids. The gas solubility in liquids is greatly affected by temperature and pressure as well as the nature of the solute and the solvent.
Many gases dissolve quickly in water, while others do not under typical conditions. Oxygen is only slightly soluble in water, whereas HCl or ammonia dissolves quickly.

Factors Affecting Solubility of Gases In Liquids
The factors affecting the Solubility of gas in Liquid are discussed below,
Effect of Pressure
It has been discovered that as pressure rises, so does the solubility of a gas in liquids. Consider a system of a gas solution in a solvent in a closed container in a state of dynamic equilibrium to better understand the effect of pressure on gas solubility. Because the solution is now in equilibrium, the rate of gaseous molecules entering it is equal to the rate of gaseous molecules leaving it. As a result, the number of gas molecules in the solution increases until a new equilibrium point is reached. As a result, the solubility of gases increases as the pressure of a gas above the solution rises.
Effect of Temperature
With increasing temperature, gas solubility in liquids decreases. Dissolution is the process by which gas molecules in a liquid dissolve. Heat is emitted throughout the process. When a system’s equilibrium is disturbed, the system readjusts itself in such a way that the effect that caused the change in equilibrium is offset, according to Le Chatelier’s Principle. Because dissolution is an exothermic process, solubility should decrease as the temperature rises, proving Le Chatelier’s Principle.
Learn more about, Factors affecting Solubility
Henry’s Law
According to Henry’s Law, the solubility of a gas in a liquid is directly proportional to the pressure of the gas at a fixed temperature. More formally, “The partial pressure of the gas in the vapor phase (p) is proportional to the mole fraction of the gas (x) in the solution,” says the most popular version of Henry’s law. Mathematically it is given as:
p = KHx
Here, KH is Henry’s Law constant
Applications of Henry’s Law
Henry’s Law is one of the most important laws used in solutions and its various applications are,
- In the manufacture of carbonated drinks.
- Climbers and those who live at high altitudes will benefit from Henry’s Law
- During a deep-water dive a diver uses Henry’s Law to use proper oxygen.
Raoult’s Law (Special Case of Henry’s Law)
According to Raoult’s law, “solubility of a gas in a liquid is directly proportional to the partial pressure of the gas above the liquid”. Mathematically it can be written as
p = xipi0
One of the components of a gas in a liquid solution is so volatile that it exists as a gas. According to Henry’s law, it is soluble in water.
p = KHx
As a result, Raoult’s law is a specific case of Henry’s law, in which KH equals pi0be dissolved
Read More,
Examples on Solubility
Example 1: At 313 K, benzene and toluene form perfect solutions A and B. 4 moles of toluene and 1 mole of C6H6 makeup Solution A. Toluene and benzene are equal amounts in Solution B. In each scenario, calculate the total pressure. At 313 K, C6H6 and toluene have vapor pressures of 160 and 60 mm, respectively.
Solution:
- For Solution A
PM = P‘B + P‘T = (P0B × XB) + (P0T × XT)
PM = 160 × (1/1+4) + 60 × (4/1+4)
PM = 32 + 48
PM = 80 mm
- For Solution B
PM = 160 × (92/170) + 60 × (78/170)
PM = 86.588 + 27.529
PM = 114.117 mm
Example 2: Heptane and octane form an ideal solution at 373 K, the vapor pressures of the pure liquids at this temperature are 105.2 kPa and 46.8 kPa respectively. If the solution contains 25g of heptane and 28.5g of octane, calculate the vapor pressure exerted by heptane.
Solution:
Given,
- Po(C7H16) = 105.2 kPa
- Po(C8H18) = 46.8 kPa
- M(C7H16) = 100g mol-1
- M(C8H18) = 114g mol-1
X{C7H16} = n(C7H16) / {n(C7H16) + n(C8H18)}
X{C7H16} = (25/100) / ((25/100) + (28.5/114))
X{C7H16} = 0.25/0.25 + 0.25
X{C7H16} = 0.5
X{C8H18} = 1 – 0.5 = 0.5
P{C7H16} = 105.2 × 0.5 = 52.60 kPa
FAQs on Solubility
1. What is Solubility in Chemistry?
Solubility is the ability of a substance (solute) to dissolve in a solvent to form a homogenous solution.
2. What Factors Affect Solubility?
The factor that affect the solubility are, Temperature, Pressure, and Chemical Nature.
3. How does Temperature Affect Solubility?
Solubility of solids in liquids increases with temperature, while the solubility of gases in liquids decreases with temperature.
4. How does Pressure Affect Solubility?
Solubility of gases in liquids increases with pressure, while the solubility of solids in liquids is generally unaffected by pressure.
5. What is Solubility Product Constant?
Solubility product constant (Ksp) is a measure of the maximum amount of a solute that can dissolve in a given solvent at a particular temperature and pressure.
6. What is the Difference between an Unsaturated, Saturated, and Supersaturated Solution?
An unsaturated solution is a solution that contains less than the maximum amount of solute that can dissolve in a solvent. A saturated solution is a solution that contains the maximum amount of solute that can dissolve in a solvent. A supersaturated solution is a solution that contains more solute than is normally possible at a given temperature and pressure.
7. What is “Like Dissolves Like”?
This means that substances with similar polarities or intermolecular forces are more likely to dissolve in each other.
8. What are Effects of Temperature and Pressure on Solubility?
An increase in pressure and temperature contributes to higher solubility in this process. An rise in pressure causes more gas particles to enter the liquid, lowering the partial pressure. As a result, the solubility will rise.
9. What is Solubility Product Constant?
Solubility Constant denoted by Ksp is the equilibrium constant that represents the extent to which a solid solute dissolves in a solution.
Vapour Pressure
Vapour pressure is the force exerted by a liquid’s (or solid’s) vapour above the surface of the liquid. At a particular temperature and thermodynamic equilibrium, this pressure is formed in a closed container. The rate of liquid evaporation is controlled by the equilibrium vapour pressure. The vapour pressure increases with increasing temperature. When atmospheric pressure and vapour pressure are equal, a liquid is said to have reached its boiling point.
Characteristics of Vapour Pressure
Vapour pressure is defined as the pressure exerted by the liquid in the thermodynamic equilibrium state. Any substance that has a high vapour pressure at normal temperature is termed a volatile substance. The vapour pressure at the equilibrium state indicates the rate of evaporation in the liquid.
In the stage of equilibrium, the pressure exerted by the atmosphere is equal to the vapour of the liquid.
Factors Affecting Vapour Pressure
Vapour Pressure of the liquid depends on the various factor discussed below:
- Nature of Liquid: Intermolecular forces between the atoms of the liquid explain the nature of the liquid. Vapour Pressure of the liquid changes according to different types of liquids.
- Effect of Temperature: With the increase in temperature of the liquid, the kinetic energy of the atoms of the liquid also increases. Now, the liquid molecules with high kinetic energy are less likely to escape thus, the vapour pressure of the liquid increases. Hence we can say that vapour pressure is directly proportional to temperature.
- Concentration of Solute: The existence of a solute in the liquid will significantly reduce the vapour pressure. And this fall in vapour pressure also differs with respect to the concentration of solute.
What is Raoult’s Law?
According to Raoult’s law, the vapour pressure of a pure component (liquid or solid) multiplied by its mole fraction in the mixture results in the partial pressure of that component in a perfect mixture of liquids. As a result, the mole fraction of solute in the solution equals the relative decrease in vapour pressure of a diluted solution of a non-volatile solute.
A solution that complies with Raoult’s law is referred to as the “perfect solution”. While some liquid combinations adhere to this law over a wide range of concentrations, this law only holds for diluted solutions. Perfect solutions are produced because the intermolecular forces between molecules of pure substances are the same as the forces between molecules of one material and molecules of another.
Raoult’s Law or Vapour Pressure Formula
When a solid is dissolved into a liquid, a solution is made. When the solute is added, the vapour pressure that results from this solution is reduced. Here is the formula for vapour pressure using Raoult’s law, which explains how the vapour pressure of a liquid changes when a solute is added.
Psolution = (Xsolvent)×(P° solvent)
where,
Psolution is the vapour pressure of a solution
Xsolvent is the mole fraction of solvent in a solution
P°solvent is the vapour pressure of a solvent
Importance of Raoult’s law
Let’s say a volatile liquid A is placed inside a closed container. After some time, vapour particles will start to develop as a result of evaporation. The liquid particles on the surface will eventually be in dynamic equilibrium with the vapour particles of A as time goes on. As a result, the pressure that A’s vapour particles are exerting at any given temperature is known as A’s vapour pressure at that temperature. All solids and liquids display vapour pressure, which is solely dependent on the kind of liquid and temperature.

The A particles will now fill the spaces between the B particles on the surface of the solution if liquid B is introduced to this container.
A portion of the molecules on the surface of any given liquid have enough energy to convert to the vapour phase.
Since there are currently fewer A particles on the surface, there will be fewer A vapour particles in the vapour phase. This will cause A’s vapour pressure to decrease.
If B is also volatile, there will be fewer B particles in the vapour phase than there would be if B were a pure liquid.

This new pressure, which is determined by Raoult’s equation and depends on the component concentration of each liquid phase, is the partial pressure of each A and B
PA ∝ XA = XAP°A
PB ∝ XB = XBP°B
P° represents the component of the mole fraction.
Vapour Pressure of Liquid-Liquid Solutions
Let’s use two volatile liquid solutions labelled 1 and 2 to calculate the vapour pressure of a liquid-liquid solution. An equilibrium between the binary solution’s liquid phase and vapour phase is created when the liquid components are added to a closed vessel.
Assume that PTotal is the total vapour pressure of the binary solution at equilibrium and P1 and P2 are the respective partial vapour pressures of components 1 and 2. Let the mole fractions of components 1 and 2 be X1 and X2, as the partial pressures are related to the mole fractions.
Vapour Pressure of Solutions of Solids in Liquids
We mix sugar and water to create a sugar solution. The non-volatile solute in this mixture is sugar, while the solvent is water. Due to the non-volatility of the solute, when this sugar solution evaporates, the vapour phase only consists of water vapours. However, it is found that this solution’s vapour pressure is lower than that of the pure solvent (water).
The rate at which the solvent molecules escape from the surface of the liquid determines the vapour pressure of a solution made up of a non-volatile solute and a solvent. As a result, the vapour pressure of the solution (sugar and water) will be lower than that of the pure solvent (water), or the addition of a non-volatile solute will cause the vapour pressure of the solution to decrease. The solution’s vapour pressure will decrease more when the concentration of sugar in the solution rises.
The amount of non-volatile solute present in the solution, not the type of non-volatile solute, determines how much of the solvent’s vapour pressure is reduced.
What is a boiling point?
Vapour pressure of any liquid increases with the increase in temperature as the temperature increases there comes a stage at which the vapour pressure of the liquid is equal to the atmospheric pressure. It reaches a stage where the vapour pressure of the liquid becomes equal to the atmospheric pressure. In this stage, the vapour of liquid escapes to the atmosphere and the temperature of the liquid at this stage is called the boiling point of the liquid.
For the standard value of the boiling point of liquid pressure is taken to be,
Pressure = 1 atm = 102325 Pa = 1 bar
What is the Heat of Vapourization?
The heat required by 1 mole of liquid to change its phase from liquid to vapour form is called the Heat of Vapourization. The heat of Vapourization only changes the phase of the liquid to gaseous form it does not increase the temperature of the liquid.
Raoult’s Law and its Relationship with Other Laws
Raoult’s Law is one of the basic laws and its relation with other laws can be stated as,
- Ideal gas law and Raoult’s law resemble one other quite a bit. Raoult’s law does not apply to solutions, which is the single exception. If you’ve read about the ideal gas law, you know that it implies that gases would behave in an ideal manner, with zero or no intermolecular forces between molecules of different types. Meanwhile, Raoult’s law makes the hypothesis that the intermolecular forces between unlike and comparable molecules are equal.
- Non-ideal solutions are similarly subject to Raoult’s law. The interactions between molecules of various substances must be taken into account when incorporating several components, though.
If we use an ideal system made up of an ideal liquid and an ideal vapour, we may also deduce a very - By combining Raoult’s law and Dalton’s law, we may further obtain a highly helpful equation for an ideal system made up of an ideal liquid and an ideal vapour.
- This equation tells us that each component of an ideal solution made up of pure substances will have a different vapour pressure. Additionally, the component will have a higher pure vapour pressure in the gas phase compared to the solution, which will have a lower pure vapour pressure.
Limitations of Raoult’s Law
Raoult’s Law has limitations which are discussed below,
- Raoult’s law is especially relevant since it refers to ideal solutions, i.e., those in which the gas phase has thermodynamic properties similar to a combination of ideal gases.
- The only drawback is that they are hard to find and rare. Different chemical components must be chemically equivalent.
- Several solutions deviate from Raoult’s law because various attractive forces exist in many liquid mixtures. So, do not properly obey it.
Also, Check,
Solved Examples on Vapour Pressure Formula
Example 1: At 25 °C, an aqueous solution’s vapour pressure is measured to be 23.80 mmHg. What fraction of the solute by a mole in this solution? At 25 °C, water has a vapour pressure of 25.756 mm Hg.
Solution:
Vapour pressure of the solution, P Solution = 23.80
Vapour pressure of solvent, P° Solvent= 25.756
Using vapour pressure formula:
Psolution = (Xsolvent) (P°solvent)
23.80= (XSolvent) 25.756
XSolvent = 0.92405
Mole fraction of Solvent = 0.92405
Mole fraction of Solute = 1 – 0.92405
= 0.07595
Example 2: At 25°C, an aqueous solution’s vapour pressure is measured at being 20mmHg. What is the mole fraction of the solvent in this solution? At 25 °C, water has a vapour pressure of 60 mm Hg.
Solution:
Vapour pressure of the solution, P Solution = 20
Vapour pressure of solvent, P° Solvent= 60
Using vapour pressure formula:
Psolution = (Xsolvent) (Posolvent)
20= (XSolvent) 60
XSolvent = 0.333
Mole fraction of solvent = 0.333
Example 3: What is the vapour pressure of the solution if the mole fraction of the solute is 0.3? Water has a 16.358 mmHg vapour pressure at 23 °C.
Solution:
Mole Fraction of solute = 0.3
Mole Fraction of solvent, XSolvent = 1 – 0.3
= 0.7
Now,
Vapour pressure of solvent, P°Solvent = 16.358
Mole Fraction of solvent = 0.7
Using vapour pressure formula:
Psolution = (Xsolvent) (P°solvent)
= (0.7)(16.358)
= 11.4506
Vapour pressure of the solution is 11.4506
Example 4: 200 grams of sucrose dissolved in 300 grams of water at 30 degrees. How much vapour pressure does this solution have? At 30.0 °C, the vapour pressure of water is 41.62 mmHg.
Solution:
First, we need to find the fraction of mole in a solvent.
Now, the amount of mole in solute
Moles of Sucrose = wt (gm)/ molecular mass (molecular weight of sucrose = 342.2948)
= 200 / 342.2948g
= 0.5843Moles of Water = 300 / 18.015 (molecular weight of water = 18.015)
= 16.6528Total mole of solution = 0.5843+16.6528 = 17.2371
Mole fraction of solvent (water) = Mole of water / Mole of solution
Mole fraction of solvent, X Solvent = 16.6528/(16.6528 + 0.5843)
= 16.6528/17.2371
= 0.9661
vapour pressure of solvent, P°Solvent = 41.62
Using vapour pressure formula:
Psolution = (Xsolvent) (P°solvent)
= (0.9661)(41.62)
= 40.2090
Vapour pressure of the solution is 40.2090.
Example 5: What is the vapour pressure of 400 g of propanol (molecular weight = 60 g/mol) and 130 g of acetone (molecular weight = 58 g/mol)? Propanol and acetone have vapour pressures of 11 and 20 mmHg, respectively, at 25 °C.
Solution:
Calculating Molar fractions:
Number of moles in acetone, nacetone = 130g / 58g/mol = 2.24 mol
Number of moles in propanol, npropanol = 400g / 60g/mol = 6.66 mol
ntotal = nacetone + npropanol = 2.24 + 6.66 = 8.9 mol
Molar fraction of acetone, xacetone= 2.24/8.9 = 0.2516
Molar fraction of propanol, xpropanol = 6.66/8.9 = 0.7483
Vapour pressure of acetone, Pacetone = 20
Vapour pressure of Propanol, P°Propanol= 11
Now, partial pressure can be calculated as:
Pacetone = (xacetone) ( P°acetone) = 0.2516 × 20 = 5.03 mmHg
PPropanol = (xPropanol) ( P°Propanol) = 0.7483 × 11 = 8.2313 mmHg
vapour Pressure of the Mixture
Pmixture = Pacetone + PPropanol
= 5.03 + 8.2313
= 13.2613 mmHg
FAQs on Vapour Pressure Formula
Question 1: What is Raoult’s law and its application?
Answer:
Raoult’s law states that the partial pressure of a pure component (liquid or solid) in a perfect mixture of liquids is equal to the vapour pressure of that component multiplied by the mole fraction in the mixture.
Raoult’s law is applicable:
- To evaluate the reduction in non-volatile solute vapour pressure.
- To evaluate the liquids’ ability to bind strongly.
Question 2: Write the Vapour Pressure formula.
Answer:
A solution is created when a solid is dissolved in a liquid. The resultant vapour pressure of this solution is decreased when the solute is introduced. Raoult’s law, which describes how the vapour pressure of a liquid changes when a solute is added, is used to calculate vapour pressure.
Psolution = (Xsolvent)×(P° solvent)
where,
Psolution is the vapour pressure of a solution
Xsolvent is the mole fraction of solvent in a solution
P° solvent is the vapour pressure of a solvent
Question 3: For what type of solutions, Raoult’s law is valid?
Answer:
Raoult’s law is only valid for ideal solutions. The solvent-solute interaction is the same as a solvent-solvent or solute-solute interaction in an ideal solution. This suggests that both the solvent and the solute expend the same amount of energy to reach the vapour phase as they do in their pure states.
Question 4: Write the limitations of Raoult’s law.
Answer:
There are limitations of Raoult’s law that are mentioned below:
Since Raoult’s law applies to ideal solutions, i.e., those in which the gas phase exhibits thermodynamic features resembling a combination of ideal gases, it is particularly pertinent. The fact that they are uncommon and hard to find is the only negative. Because distinct attractive forces exist in many liquid mixes, certain solutions depart from Raoult’s equation.
Colligative Properties
Colligative Properties of any solution is the property of the solution that depends on the ratio of the total number of solute particles and the total number of solvent particles. Changing the moles or number of particles of solute or solvent changes the colligative properties of the solution. These colligative properties are not dependent on the chemical nature of the solute or solvent but are rather dependent on the number of solutes and solvent particles in the solution. These colligative properties depend on the number of particles in the solution rather than the nature of the solute and the solvent. These properties can be easily linked with the concentration of the solution, i.e. Molarity, Normality, and Molality.
In this article, we will learn about, various types of Colligative properties of the solution, their examples, and others in detail.
Table of Content
What are Colligative Properties?
Colligative Properties are taken from the Greek word “Colligatus” which means “bound together”. The colligative properties of the solution, are the properties that are bound by the number of solute and solvent particle present in the solution. There are four types of colligative properties exhibited by the solution, that are,
- Elevation of Boiling point
- Depression Freezing point
- Lowering of Vapour Pressure
- Osmotic Pressure
Colligative Properties Definition
The properties of the dilute solution in which a non-volatile solute is added, that depends on the number of the solute particle rather than the chemical nature of the solvent are called the colligative properties of the solution.
Colligative properties of any solution depends on the solute-solvent mass ratio of the solution and is independent of the nature of the solute and the solvent. Colligative properties are inversely proportional to the molar mass of the solute.
Colligative Properties Examples
Various example of the colligative properties can be easily seen in our daily life. Such as when we add some salt in the water the freezing point of the water increases, i.e. it freezes far below its normal freezing point. Also, the boiling point of the water also increases if we add salt or sugar in the water. Similarly adding alcohol to water decreases the freezing point of the water.
As these colligative properties are dependent on the concentration of the solution, let’s first learn about the measure of concentration of the solution.
Molarity (M)
Molarity is the concentration of a solution, measured as the number of moles of solute per liter of solution. Molarity of any solution is calculated by the formula,
Molarity = Number of Moles of Solute/Volume of Solution in L
Molality (m)
Molality is the amount of a substance dissolved in a certain mass of solvent. It is defined as the number of moles of a solute per kilogram of a solvent. Molality of any solution is calculated by the formula,
Molality = Number of Moles of Solute/Weight of Solvent in Kg
Mole Fraction (x)
Mole fraction of a solute in a solution gives the ratio of the number of moles of the solute present in the solution to the total number of moles of the solute and the solvent present in the solution. There is another definition for mole fraction that is, “Mole fraction of a compound is the ratio of the number of moles of the compound to the total moles of compounds in the mixture.” Mole fraction of any solution is calculated by the formula,
Mole Fraction = (Number of Moles of Solute)/(Total Number of Moles of Solute and Solvent)
Types of Colligative Properties
There are in general four types of the collugative properties of any solution that are mentioned below
- Lowering Of Vapour Pressure
- Elevation in Boiling Point (Boiling Point Elevation)
- Depression in Freezing Point (Freezing Point Depression)
- Osmotic Pressure.
Now let’s learn about them in detail.
Lowering Of Vapour Pressure
Lowering of vapour pressure is the ratio of vapour pressure of the solution to the vapour pressure of the pure solvent. If we add any non-volatile solute to the solvent we can easily lower the vapour pressure of the solution. the image added below shows the relative lowering of the vapour pressure of any solution.

Suppose if Po is the vapour of the pure solvent and Ps is the vapour pressure of the solvent then relative lower of the vapour pressure is calculated by taking the ratio of (Po – Ps) and Po. i.e.
Relative Lowering of Vapour Pressure = (Po – Ps)/Po
The relative lowering of the vapour pressure of a solution having non-volatile solutes is equal to the mole fraction of the solute. If n is the number of moles of the solute and N is the number of moles of the solvent. Then we can say that,
(Po – Ps)/Po = n/(n + N)
Elevation in Boiling Point (ΔTb)
Boiling point of the liquid is the temperature at which the vapour pressure of the liquid is equal to the atmospheric pressure. If we add any solute to the solvent the boiling point of the solution is greater than the boiling point of the solvent.
Elevation of the boiling point is the difference between the boiling point of the solution and the boiling point of the solvent. If Tb is the boiling point of pure solvent and TIb is the boiling point of the pure solvent. Then elevation of boiling point is given as,
ΔTb = TIb – Tb
Experimentally it is proven that, ΔTb is proportional to morality of the solute, then
ΔTb ∝ m
ΔTb = kbm
Where, kb is molar elevation constant.
ΔTb = 1000kb(m2)/(M2m1)
where,
- m2 is the mass of solvent in g
- m1 is the mass of solvent in kg
- M2 is the molar mass of solute
Depression in Freezing Point (ΔTf)
Freezing point of the liquid is the temperature at which the vapour pressure of the substance in its liquid phase is equal to its vapour pressure in the solid phase. If we add any solute to the solvent the freezing point of the solution is lower than the freezing point of the solvent.
Depression of frezzing point is the difference between the frezzing point of the solution and the freezing point of the solvent. If Tf is the frezzing point of pure solvent and TIf is the frezzing point of the pure solvent. Then deprezzion in freezing point is given as,
ΔTf = TIf – Tf
Experimentally it is proven that, ΔTf is proportional to morality of the solute, then
ΔTf ∝ m
ΔTf = kfm
Where, kf is molal elevation constant.
ΔTf = 1000kb(m2)/(M2m1)
where,
- m2 is the mass of solvent in g
- m1 is the mass of solvent in kg
- M2 is the molar mass of solute
Osmotic Pressure (π)
Osmotic Pressure is the colligative property of the solution and is defined as the difference needed to stop the flow of solvent across the semipermeable membrane.
We can also define the Osmotic Pressure as the minimum pressure that needs to be applied to a solution to prevent the inward flow of its pure solvent across a semipermeable membrane. It is also defined as the measure of the tendency of a solution to take in a pure solvent by osmosis.
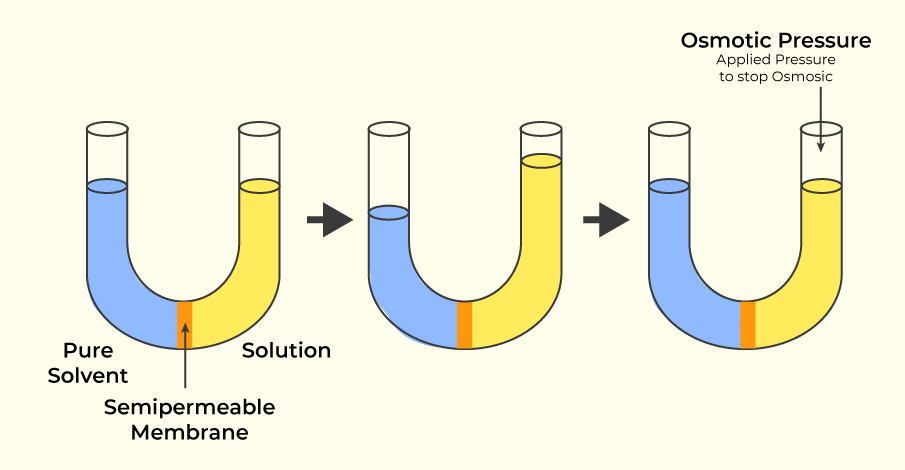
Osmotic Pressure is calculated by the formula,
π = CRT
Where,
- C is Concentration (or) Molarity
- R is Universal Gas Constant
- T is temperature
Types of Solutions
Different Types of the solution are,
- Isotonic Solution
- Hypotonic Solution
- Hypertonic Solution
Isotonic Solution
Isotonic Solutions are the solutions that have same osmotic pressure at any given temperature. No osmosis happens in case of Isotonic Solution.
Hypotonic Solution
If a solution have lower osmotic pressure then the surrounding then it is called Hypotonic Solution.
Hypertonic Solution
If a solution have higher osmotic pressure then the surrounding then it is called Hypertonic Solution.
Van’t Hoff Factor
For a solute that dissociates in the solution its properties changes by a factor and this factor is called the Von’t Hoff Factor. Von’t Hoff Factor is denoted using(i). It is calculated as,
- i = (Observed Colligative Properties)/(Theoretical Colligative Properties)
- i = (No. of Particles after Dissociation Or Association)/(No. of Particles is case of no Dissociation Or Association)
Raoult’s Law
Raoult’s law states that, “Vapour pressure of a pure component (liquid or solid) multiplied by its mole fraction in the mixture results in the partial pressure of that component in a perfect mixture of liquids. As a result, the mole fraction of solute in the solution equals the relative decrease in vapour pressure of a diluted solution of a non-volatile solute.
Raoult’s Law states that,
Psolution = (Xsolvent)×(P° solvent)
where
- Psolution is Vapour Pressure of a Solution
- Xsolvent is Mole Fraction of solvent in a Solution
- P°solvent is Vapour Pressure of a Solvent
Read More,
Solved Examples on Colligative Properties
1. Find the relative lowering of vapour pressure, if 18 g of glucose is dissolved in 90 g of water.
Solution:
Given,
- Mass of glucose = 18 g
- Mass of water = 90 g
Molar Mass of Glucose = 180 g/mol
Number of moles of glucose(nB) = 18/180 = 0.1
Molar Mass of Glucose = 18 g/mol
Number of moles of water(nA) = 90/18 = 5
Relative lowering ΔP/PA° is equal to XB
XB = nB/(nA + nB)
XB = 0.1/0.1 + 5 = 1/51
Relative Lowering of Vapour Pressure(ΔP/PA°) = 1/51
2. Boiling point elevation of a solution containing sucrose and water is 0.256 °C. The molal elevation constant of water is 0.512 °C/m and molar mass of sucrose is 342 g/mol. What is molality?
Solution:
Given:
- Boiling point elevation (ΔTb) = 0.256 °C
- Molal elevation constant (Kb) = 0.512
(ΔTb) = Kb × m
0.256 = 0.512 × m
m = 0.5 mole/kg
3. Calculate the freezing point depression and the freezing point after adding 100.0 g of table salt to 400.0 g of water. (Kf of water = 1.86)
Solution:
Moles of NaCl = mass/molar mass
Moles of NaCl = 100.0/58.443 = 1.71107 mol
Mass of water = 400.0 g = 0.400 kg
Molalilty(m) = (moles of NaCl)/(mass of water in kg)
m = 1.71107/0.400 = 4.2777
NaCl ⇢ Na+ + Cl–
Van’t Hoff factor(i) = Numberof mole after dissociation/number of mole before dissociation
i = 2
Freezing point depression constant for water Kf = 1.86
Freezing point depression = i × Kf × m = 2 × 1.86 × 4.2777 = 15.9 °C
Freezing point of solution = (freezing point of water – freezing point depression) = 0.0 – 15.9
Freezing point of solution = -15.9 °C
4. If 6.8% w/v of cane sugar is isotonic with 1.52% w/v with Thiocarbamide if the molecular weight of cane sugar is 342 find the molecular weight of Thiocarbamide?
Solution:
In Isotonic solution,
π1 = π2 = i2C2RT
Now, i1C1 = i2C2
For canesugar and thiocarbamide are non electrolytes,
So i = 1
Thus, C1 = C2
% (W/V) percent is the number of grams of solute in 100 mL of solution
C = (Number of moles of solute)/(Volume of solution in L)
C1 = 6.8 × 1000/342 × 100
C2 = 1.52 × 1000/x × 100
As C1 = C2
6.8 × 1000/342 × 100 = 1.52 × 1000/x × 100
x = 76
Thus, weight of Thiocarbamide is 76 g
5. Osmotic pressure, of a solution of glucose, is 117.4 atm. Find the molarity of the solution at 298 K.
Solution:
π = iCRT
(Glucose is a Non Electrolyte, i = 1)
117.4 = C × 0.0821 × 298
C = 4.8 mole/l
Thus, the concentration of glucose is 4.8 moles/litre
6. x grams of solute is dissolved in 500 gram solvent if the sum of elevation of boiling point and depression in freezing point for sucrose in water is 5 find its molality. (if Kb = 0.52 and Kf = 1.86) find x?
Solution:
ΔTf + ΔTb = 5
We now that,
- ΔTf = i × kf × m
- ΔTb = i × kb × m
Here, i = 1 (for nonelectrolyte like sucrose)
kf × m + kb × m = 5
2.38 × m = 5
m = 2.1 mole/kg
Molality = Number of Moles of Solute/Weight of Solvent in Kg
(x × 1000)/(342 × 500) = 2.1
x = 359 gram
Thus, the solute dissolved is 359 grams.
Colligative Properties FAQs
What are colligative properties class 12?
The colligative properties of the solution are the properties that are dependent on the number of solute added in the solution.
Why are colligative properties important?
Colligative Properties of any solution are important for studying various natural phenomenon and designing various apparatus.
Are colligative properties physical or chemical?
The physical changes that occurs when the solute particle is added to any solvent are called the colligative properties of the solution. These properties only depend of the number of mole of the solute but not on the nature of the solute particles.
What are the 4 colligative properties?
The Four colligative properties of the solution are,
- Elevation of Boiling point (boiling Point Elevation)
- Depression Freezing point (Freezing Point Depression)
- Lowering of Vapour Pressure
- Osmotic Pressure
On which factor Colligative Properties Depends?
Colligative Properties Depends on the number of solute particle present in the solution
Osmosis and Osmotic Pressure
A solution is a homogeneous mixture of two or more particles having particle sizes smaller than one nanometer. Sugar and salt solutions in water, as well as soda water, are common examples of solutions. In a solution, all of the components appear as a single phase. There is particle homogeneity, which means that the particles are evenly dispersed. This is why a full bottle of soft drink tastes the same.
The component that dissolves the other component is known as the solvent. Solute refers to the component(s) that are dissolved in the solvent (s). In general, the solvent is present in greater proportion than the solute. The solute amount is less than the solvent amount. Solutes and solvents can exist in every state of matter, including solids, liquids, and gases. A liquid solution is composed of a solid, liquid, or gas dissolved in a liquid solvent. Solid and gaseous solutions are represented by alloys and air, respectively.
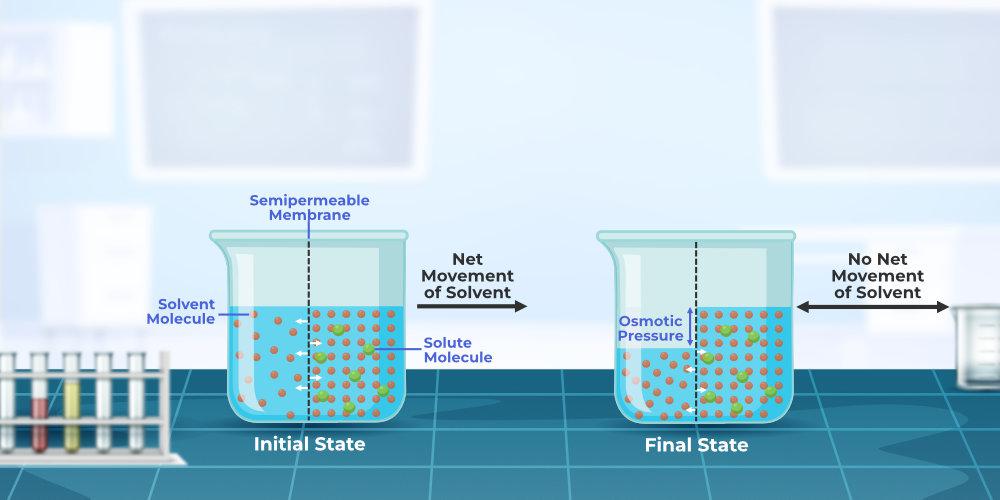
Osmosis
Osmosis is the process of moving solvent molecules from a low solute concentration area to a high solute concentration area through a semipermeable membrane. Eventually, an equilibrium between the two sides of the semipermeable membrane is formed (equal solute concentration on both sides of the semipermeable membrane). Because the semipermeable barrier only allows solvent molecules to pass through, no solute particles can pass through.
Note: Osmosis is discovered and named by the French physiologist Henri Dutrochet. He also invented osmometer, a device used to measure osmotic pressure.
Osmotic Pressure
Osmotic Pressure is the least pressure required if applied to a solution, the inward flow of solvent molecules across the semipermeable membrane is stopped. It is a colligative property that is regulated by the concentration of solute particles in the solution.
Osmotic Pressure Formula
The Dutch chemist Jacobus van’t Hoff proposed this link between a solution’s osmotic pressure and the molar concentration of its solute. which is as follows:
∏ = iCRT
Where,
∏ is the osmotic pressure,
i is the van’t Hoff factor,
C is the molar concentration of the solute in the solution, (C=n/V; where n is the number of moles and V is the volume.)
R is the ideal gas constant,
and T is the temperature in Kelvin
It should be noted that this equation only applies to solutions that act like perfect solutions.
Reverse Osmosis
The minimal pressure required to stop the passage of the solvent across the semipermeable membrane is referred to as osmotic pressure. When a pressure greater than the osmotic pressure is applied to the solution side (the side with a high solute concentration), the solvent particles on the solution side move through the semipermeable membrane to the area with a low solute concentration. Reverse osmosis refers to the flow of the solvent through the semipermeable membrane in the opposite direction.
Application of Reverse Osmosis
- Electronic component manufacturers require the finest quality water possible. Reverse osmosis is commonly used to remove the majority of contaminants from a water supply before it is introduced into a polishing ion exchange system. Reverse osmosis increases the life of the ion exchange beds while lowering the overall cost of producing huge volumes of high-quality water.
- Depending on the nature of the chemical production process, the maker of chemicals requires varied grades of water. In some circumstances, reverse osmosis water will yield satisfactory product water on its own, and it is utilized as a pre-treatment when greater qualities are required.
- In this business, reverse osmosis has been used successfully to not only purify water for use in plating solution makeup water and drag out baths but also to concentrate important plating metals in the waste stream for recycling in a closed-loop process.
- On a small and large scale, reverse osmosis is widely utilized in the desalting sea or brackish water for potable consumption. Because of its low energy requirements, the technique is particularly appealing in this application.
Types of Osmosis
There are two types of Osmosis that take place in the cells of animals as well as plants, those are as follows:
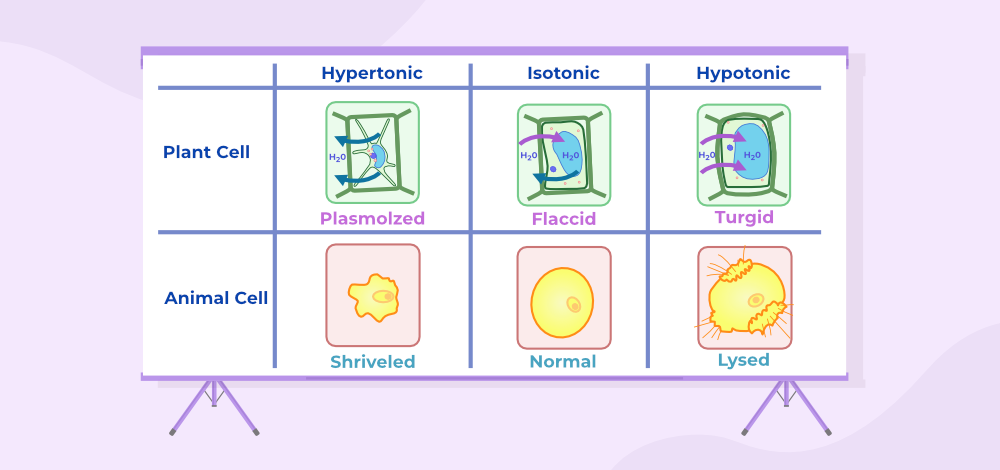
Endosmosis
When a substance is immersed in a hypotonic solution, the solvent molecules migrate into the cell, creating turgidity (swollen) or deplasmolysis. This process is called Endosmosis.
Exosmosis
When a material is immersed in a hypertonic solution, the solvent molecules escape the cell, causing flaccidity or plasmolysis. This process is called Exosmosis.
Effect of Osmosis on Cells
In biological, systems osmosis is very essential as many biological membranes are semipermeable. For example in an animal cell, if surrounded by a hypertonic environment (outside the cell is higher water concentration) then due to osmosis water leaves the cell and the cell shrinks, opposite to it if surrounded by the hypotonic surroundings (outside the cell with lower water concentration) then water diffuses into cells and causes the cell to swell. Animal cells can only live if it is surrounded by an isotonic solution. The same effect of hypertonic and hypotonic solutions can be seen in plants cell.
Difference between Osmosis and Diffusion
Osmosis can seem like diffusion but there are a lot of differences between both which are as follows:
Osmosis | Diffusion |
|---|---|
| It is only applicable to liquid media. | It can be found in a variety of liquids, gases, and even solids. |
| A semipermeable membrane is required. | Doesn’t require a semi-permeable membrane. |
| This is determined by the number of solute particles dissolved in the solvent. | It is affected by the presence of other particles. |
| Water is required for particle mobility. | The mobility of particles does not require the use of water. |
| Only the solvent molecules can diffuse. | Solute and solvent molecules can both disperse. |
| Particles can only flow in one direction. | The movement of particles occurs in all directions. |
| The entire process can be stopped or reversed by applying extra pressure to the solution side. | This process cannot be halted or reversed. |
| This only happens amongst solutions that are similar in nature. | Occurs between solutions that are similar and solutions that are dissimilar. |
| Only water or another solvent goes from a high-energy or concentration zone to low energy or concentration region. | Any substance can migrate from a location of high energy or concentration to a region of low energy or concentration. |
Significance of Osmosis
- Nutritional supply and the discharge of metabolic waste products are both affected by osmosis.
- It is in charge of absorbing water from the earth and transporting it to the plant’s higher portions via the xylem.
- It maintains the equilibrium of water and intercellular fluid levels in a living organism’s interior environment.
- It keeps the turgidity of cells.
- It is the method by which plants maintain their water content in the face of continual water loss owing to transpiration.
- This process regulates water transport from cell to cell.
- Osmosis causes cell turgor, which regulates plant and plant component mobility.
- Osmosis is also responsible for the dehiscence of fruits and sporangia.
- Higher osmotic pressure protects plants against drought damage.
Examples of Osmosis
There are a lot of examples of Osmosis in nature as Osmosis is a very essential part of life. Some of the examples are as follows:
- The process through which water is absorbed from the soil is also because of osmosis as water rushes into the roots because plant roots have a higher concentration than soil.
- Osmosis also impacts the plant’s defense cells. The guard cells enlarge and the stomata open as water enters the plant cells.
- A freshwater or saltwater fish dies as a result of water entering or departing the animal’s cells when placed in water with different salt concentrations.
- Humans suffering from cholera are also affected by osmosis as the overpopulation of bacteria in the intestines reverses the absorption flow and prevents the intestines from absorbing water, resulting in dehydration.
Solved Examples of Osmotic Pressure
Question 1: Calculate the osmotic pressure of 5% solution of cane sugar (sucrose) at the temperature of 15° Celsius.
Solution:
m = molecular mass of sucrose (C12H22O11) = 342 amu
w = 5g
V = 100 mL = 0.1 litre
we know, R = 0.0821 L⋅atm⋅K−1⋅mol−1,
T = (15 + 273) = 288 K
and as glucose is the non-ionic compound and doesn’t dissociate to give any ions in the solution, it’s van’t Hoff factor is 1.
Rearranging ∏ = iCRT, we get ∏V = w/m ⋅RT,
∏ = 5/342×1/0.1 × 0.082 × 288 = 3.453 atm
Question 2: The solution containing 10 g of an organic non-ionic compound per liter showed an osmotic pressure of 1.16 atmosphere at 0° Celsius. Calculate the molecular mass of the compound (S = 0.0821 L⋅atm⋅K−1⋅mol−1)
Solution:
As compound is non-ionic, it’s van’t Hoff factor is 1.
Applying the equation m = w/∏V ⋅RT
Given w = 10 g, P = 1.18 atm, V = 1 litre, S = 0.0821 L⋅atm⋅K−1⋅mol−1 and T = 273 K.
m = 10/1.18×1 × 0.0821 × 273 = 189.94 amu
FAQs on Osmosis and Osmotic Pressure
Question 1: What is Osmosis?
Solution:
Osmosis is the process of moving solvent molecules from a low solute concentration area to a high solute concentration area through a semipermeable membrane. Some examples of osmosis are the swelling of resins when left in water for some time, the pruning of fingers after putting them in water for some time, etc.
Question 2: What is reverse osmosis?
Solution:
Reverse osmosis is a natural phenomena that takes place in the opposite direction of natural osmosis. This type of osmosis is used to remove the bulk of pollutants from water by forcing the water through a semi-permeable membrane under pressure.
Question 3: How many types of Osmotic solutions?
Solution:
There are three kinds of osmotic solutions: isotonic, hypertonic and hypotonic.
A pair of two solutions with same osmotic pressure at a given temperature are called isotonic solutions.
For two solutions, one with higher osmotic pressure and one with lower osmotic pressure compared to each other, solution with higher osmotic pressure is hypertonic solution with respect to the other solution and solution with lower osmotic pressure is called hypotonic solution with respect to the other solution.
Question 4: How is osmosis different from diffusion?
Solution:
Osmosis is the movement of solvents through a semi-permeable membrane from a low-solute-concentration region to a high-solute-concentration region. Diffusion, on the other hand, does not require a semi-permeable membrane to occur, as molecules migrate from a location of higher concentration to a region of lower concentration.
Question 5: What is a semipermeable membrane?
Solution:
The semipermeable membrane is a type of biological membrane that allows some molecules or ions to pass through it and blocks the remaining of them.
Abnormal Molar Masses
In chemistry, abnormal molar masses occur when the molar masses are estimated and are higher or lower than the predicted value. The colligative qualities are used to calculate these. Elevation of boiling point, decreased relative vapour pressure, freezing point depression, and alleviation of osmotic pressure are all colligative properties. The word abnormal is in the name, implying that the way molar masses are computed, using the Van’t Hoff factor, is abnormal. Let’s have a look.
Abnormal Molar Masses
The mole masses determined by these methods do not agree with expected or theoretical values. The exact value of the molar mass can be obtained only if the following two conditions are met.
- The solutions should be diluted: The solutions used to measure the colligative properties should not be too concentrated. In concentrated solutions, the particles interact with each other as well as with the solvent. As a result, vapour pressure and, therefore, other conjugate properties depend on the nature of the solute, not just the number of solute particles.
- The solute required is not separate or collaborative in the solution: The derivative equations for measuring colligative properties are for non-electrolyte solutes that do not undergo any dissociation or have an association solution. However, discrepancies in the determination of the molar mass arise when solutes dissociate or associate with dissolution in a solvent. This is because the number of molecules in a solution changes due to the addition or dissociation of solute molecules. Therefore, abnormal molar masses are obtained as discussed below:
For substances that undergo association, dissociation, etc. in solution, the molecular mass determined by the conjugate properties differs from the expected value. This is known as abnormal molecular mass. This can be known by the Van’t half factor.
Hence, When computed from the colligative properties of solutions, the theoretical values of molecular mass are sometimes found to differ from the empirically measured values known as the Abnormal molar masses.
Association of solute particles
In some solvents, usually non-polar, solute molecules undergo bonding, that is, two, three, or even more molecules interact with each other to form larger molecules. For example, suppose that n simple molecules combine to form an associated molecule:
nA ⇆ An
(single molecules) (one molecule)
Accordingly, the total number of molecules in the solution becomes less than the number of molecules of the substance added, and, therefore, the covalent properties will be less. Since the colligative properties are inversely proportional to the molar mass of the solute, in such cases the molar mass exceeds the theoretical values. For example, in benzene solvent, both ethanoic acid (acetic acid) and benzoic acid exist as dimers:

The molar masses of dimers ethanoic acid and benzoic acid are about 120 and 244 which are almost twice their normal values of 60 and 122, respectively. The bonding of solute molecules in a solution is normal due to the hydrogen bonding between these molecules. By way of illustration, benzoic acid and ethanoic acid (acetic acid) exist as dimers due to the fabrication of hydrogen bonds.
Dissociation of solute molecules
Molecules of electrolytes (acids, bases, and salts) separate or ionize in a vent to give two or more particles. For example, AB dissociates to give a double amber of particles:
AB ⇆ A+ + B–
As a result, the total number of particles in the solution increases, and hence, the colligative properties of such solutions will be large. Since the colligative properties are inversely proportional to the molar mass, the observed molar mass will be less than the theoretical value. For example, KCI, K . set aside to give ed Cl-ions.
KCI ⇆ K+ + Cl–
This means that if we dissolve 1 mol of KCl (74.5 g) in water, we expect 1 mol of K and 1 mol of Cl– ions in the solution. So instead of 1 mole of the solution, there will be 2 moles of particles. As a result, the collateral properties will also be almost twice as high as expected. For example, if E ignores interionic attraction, 1 mol of KCl in 1 kg of water will increase the boiling point by 2 x 0.52 K (K = 0.52 Km¹) = 1.04 K. Distinctly, the molar mass of the salt should be about half of its normal value, i.e. 37.25.
Van’t Hoff Factor
Van’t Hoff factor is defined as the ratio of the normal molar mass to the observed molar mass (or abnormal molar mass) of the solute, that is, i= Normal molar mass/Observed (or abnormal) molar mass
- In the case of an association, the observed molar mass is more than the normal, the factor T has a value less than 1.
- In the case of dissociation, the Van’t Hoff factor is greater than 1 because the observed molar mass has a lower value.
- In the case of solutes that do not undergo any association or dissociation in a solvent, Van’t Hoff factor will be equal to 1 because the observed and normal molar masses will be the same.
Since the molar mass is inversely proportional to the colligative property, Van’t Hoff factor can also be defined as the ratio of the observed value of the colligative property to the ordinary value of the colligative property.
i = Observed value of the colligative property / Normal value of colligative property
or
i = Total number of moles of particles after association or dissociation / Total number of moles of particles before association or dissociation
If i > 1, solute undergoes dissociation, and if i < 1 , solute undergoes association.
- For association,
where, n = number of particles associated or dissociated.
- For dissociation,
Van’t Hoff factor will always be greater than 1 for dissociation solutes. The Van’t Hoff factor is set at 1 for particles that show neither association nor dissociation. Thus, after incorporating Van’t Hoff’s factor, the colligative properties equation will be,
- Inclusion of Van’t Hoff factor modifies the equations for colligative properties as follows:
- Relative lowering of vapour pressure of solvent, (p1° -p1)/p1° = i(n2 /n1)
- Elevation in boiling point, ΔTb = iKbm
- Depression in freezing point, ΔTf = iKfm
- The osmotic pressure of the solution, π = (in2/V) RT
Sample Questions
Question 1. Why is great care taken in injecting intravenously to the same concentration as in vegetables and in a solution of blood plasma?
Answer:
During intravenous injection, the concentration of the solution for injection should be equal to that of blood plasma. If the solution is less concentrated, its osmotic pressure will be lower. Water will try to get into the red blood cells through the cell walls. As an outcome, the cells will swell and burst. On the other hand, if the solution is more concentrated, the water in the cells will try to move into the more concentrated solution outside the cell by osmosis. This causes the cells to shrink and consequently stop functioning.
Question 2. Which colligative property is privileged for characterizing the molar mass of macromolecules?
Answer:
Osmotic pressure premeditate is preferred to complete all other conjugate properties because-
- Even in dilute solutions, the osmotic pressure values are very high and can be measured accurately.
- Osmotic pressure can be premeditated at room temperature. On the other hand, the height in boiling point is measured at the higher temperature where the solute can dissociate. The depression in freezing point is measured at low temperature.
Question 3. If 0.1 mol of sugar or 0.1 mol of glucose is dissolved in one litre of water, will the depression in the freezing point be the same or different?
Answer:
The depression in the freezing point will be the same in both the solutions as both are non-electrolyte and give an equal number of solute particles.
Question 4. The outer hard shells of the two eggs are removed. One egg is placed in pure water and the other is placed in a saturated solution of sodium chloride. What will be seen and why?
Answer:
An egg placed in water will swell due to the osmosis of pure water in the egg. On the other hand, an egg placed in a saturated solution of NaCl will shrink due to the osmosis of water from the egg. This is because osmosis always occurs from a high concentration of solvent to a low concentration of solvent.
Question 5. Why is it advised to mix ethylene glycol with water in the radiator of a car while driving at a hill station?
Answer:
Ethylene glycol lowers the freezing point of water and hence, it does not freeze in a hill station.
Question 6. A solution of sodium chloride freezes at a temperature lower than water but boils at a temperature higher than water, explain.
Answer:
The freezing point of a liquid decreases upon the addition of a non-volatile solute and, therefore, a solution of sodium chloride freezes at a temperature lower than the freezing point of water. The addition of a non-volatile solute as a replacement increases the boiling point and results in the boiling point of a sodium chloride solution.
Question 6. Are equatorial solutions of sodium chloride and urea isotonic? Why?
Answer:
Sodium chloride dissociates to two ions (Na and Cl) and exerts approximately twice the osmotic pressure of 170 urea (which is non-electrolyte).

0 Comments